Share this
QT9 Unveils eQMS Enhancements for Efficiency & Compliance
by QT9 Software on August 06, 2025
QT9 Software today announced the launch of Version 17 of its electronic Quality Management System (eQMS) platform, packed with powerful new features and refinements designed to improve user efficiency, simplify compliance and enhance collaboration. These updates underscore QT9's commitment to continuous improvement and user-driven innovation.
Key Highlights of Version 17
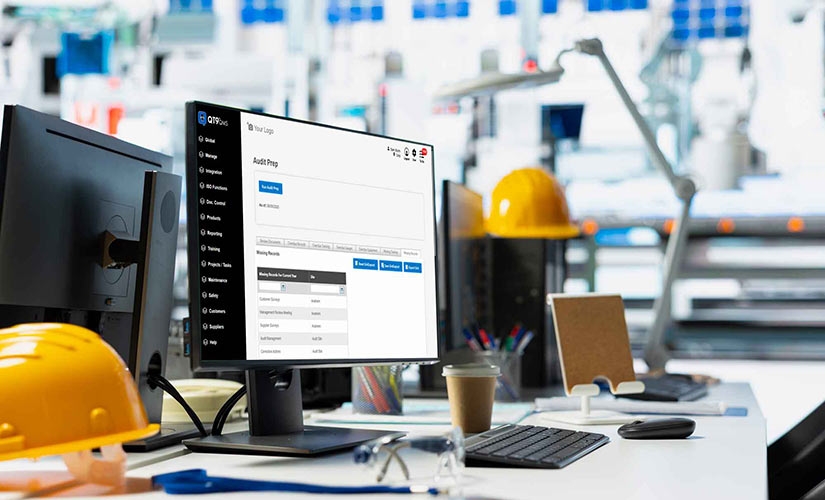
New Audit Prep Module
Say goodbye to pre-audit stress. The new Audit Prep module automatically catalogs all overdue or missing records that need your attention. This includes incomplete corrective actions, nonconforming products, deviations, missing employee training and equipment overdue for maintenance. Plus, a customizable grid allows you to tailor visibility and reporting for maximum impact.
Site-Specific Date Format Control
QT9 QMS now supports site-specific date formats, helping global teams display dates in ways that match local preferences. Month names can appear as abbreviations (e.g., DEC instead of 12) for improved clarity. Time displays follow each site’s standard settings for consistency.
More Training Integrations
Streamline your employee compliance and onboarding processes. QT9 QMS Version 17 now allows users to create training records directly from quality events or corrective actions, ensuring a smoother workflow.
Expanded Safety Reporting
The QT9 QMS Quality Events module just got more powerful. It now supports Incident Report creation, transforming the Quality Events module into a centralized hub for launching and managing all of your safety and compliance initiatives.
Single Sign-On (SSO) Enhancements
We’ve made secure access even easier. SSO is now available for both the Document Portal and the Employee Portal, simplifying logins and boosting overall accessibility.
Parallel Document Collaboration
Beyond the traditional Waterfall process, teams can now collaborate on documents in parallel, significantly improving turnaround time and offering greater flexibility.
New Change Control Capabilities
Gain more robust change management. Within Change Control records, users can now initiate risk assessments, adding to the existing deviation and corrective action options.
"These updates reflect our ongoing commitment to simplifying compliance, streamlining quality processes and empowering teams with tools that work the way they do," said Brant Engelhart, President of QT9 Software. "From audit readiness to enhanced document collaboration, every feature in this release was built to drive meaningful productivity and real results for our customers."
For a complete list of product upgrades, please contact QT9 representatives at sales@qt9software.com.
About QT9 Software
QT9 Software empowers thousands of the world’s best brands to ensure the quality and safety of their products and services through its eQMS software, ERP software, and related solutions. QT9 QMS is a validated, highly-rated solution with more than 25 standard modules, offering unlimited scalability. Its user-friendly platform enables quick implementation and accessibility with cloud-based and on-premise hosting options available. For more information, visit the QT9 Software website at https://qt9software.com.
Share this
- QT9 QMS (42)
- QT9 ERP (27)
- Manufacturing (15)
- QT9 MRP (14)
- Company News (12)
- Medical Devices (12)
- FDA Compliance (9)
- Pharmaceuticals (7)
- Inventory Management (6)
- Life Sciences (6)
- Document Control (5)
- QMSR (5)
- Aerospace & Defense (4)
- Analytics & Reporting (4)
- ISO 9001 (4)
- Supplier Quality Management (4)
- Bill of Materials (3)
- CAPA (3)
- FDA 21 CFR 820 (3)
- AS9100 (2)
- Accounting (2)
- Change Control (2)
- Electronic Batch Records (EBR) (2)
- ISO 13485 (2)
- Inspections (2)
- Audit Management (1)
- Calibration Management (1)
- Cannabis (1)
- Continuous Improvement (1)
- Cosmetics (1)
- Cybersecurity (1)
- DHF/DMR/DHR (1)
- Defense (1)
- Design Controls (1)
- EMS (1)
- EU Compliance (1)
- Employee Training (1)
- Food & Beverage (1)
- ISO 14001 (1)
- MoCRA (1)
- Quality Culture (1)
- Quality Events (1)
- Returns Management (1)
- Risk Management (1)
- Traceability (1)
- February 2026 (3)
- January 2026 (8)
- December 2025 (6)
- November 2025 (8)
- October 2025 (7)
- September 2025 (8)
- August 2025 (8)
- July 2025 (6)
- June 2025 (7)
- May 2025 (5)
- April 2025 (2)
- March 2025 (4)
- February 2025 (4)
- January 2025 (6)
- December 2024 (4)
- November 2024 (4)
- October 2024 (5)
- September 2024 (3)
- August 2024 (3)
- July 2024 (3)
- June 2024 (5)
- May 2024 (2)
- April 2024 (3)
- March 2024 (3)
- February 2024 (5)
- January 2024 (1)