Product Design Controls for End-To-End Compliance
Accelerate innovation and maintain compliance with automated, traceable design control workflows.
Accelerate Approvals
Speed up product development with design review workflows.
Ensure Full Traceability
Track design inputs, outputs and changes in one secure system.
Simplify Compliance
Maintain FDA and ISO design control requirements effortlessly.
Collaborate in Real Time
Connect teams with centralized design files and instant updates.
Reduce Risk Early
Identify and resolve design issues before they escalate.
Link to Risk & CAPA
Seamlessly connect design data with risk and corrective actions.
What is design control software?
Design control is the structured process used by manufacturers, especially in regulated industries, to manage the development and lifecycle of products. It ensures that design inputs are met with validated outputs, changes are documented and quality standards (such as ISO 13485 or FDA 21 CFR Part 820) are maintained.
QT9’s Design Control software helps automate every phase of product development—from concept to completion. Easily manage design inputs, outputs, reviews, risk assessments and approvals. Fully integrated into QT9 QMS, it simplifies compliance, enhances traceability and accelerates innovation with real-time collaboration tools.
Interactive Video Demo

QT9 QMS Design Control Demo
QT9 QMS is a fully-validated platform
We do all the software validation for you. No extra charge.
Centralize design control data
-
Eliminate data silos: Work in one system for accurate traceability and compliance.
-
Template your process: Create templates for any design control process – including Design History Files (DHFs) and ISO 9001 design controls.
-
Create custom phases: Organize phases of design and development however works best for you.
-
See design plans your way: Customize design plans with the most relevant information for your team.
-
Manage Technical Files: Centralize Device History Records (DHRs), Design History Files (DHFs) and Device Master Records (DMRs).
-
Connect your data: Link multiple QT9 QMS modules per phase, including document control, ECR, ECN, FMEA, risk assessments and inspections.
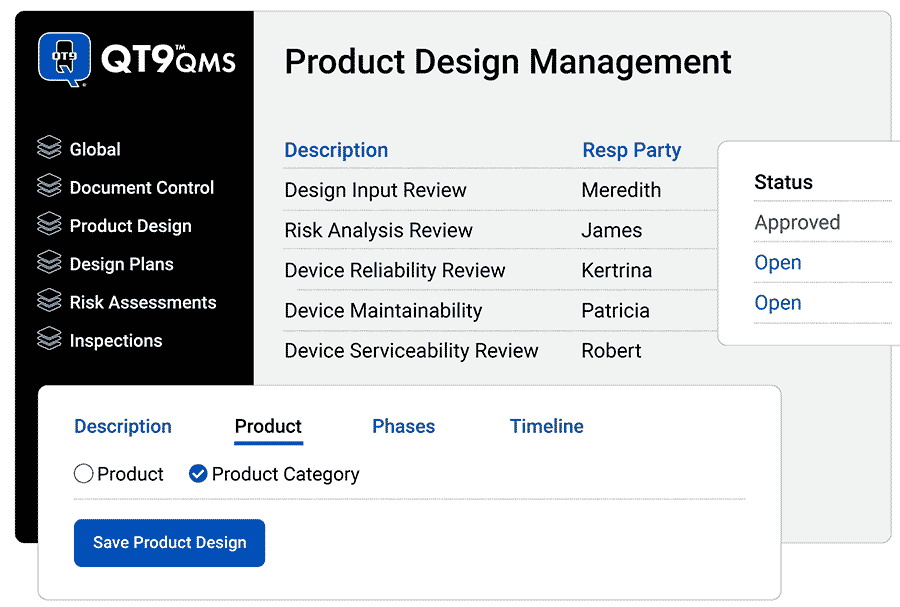
Get total traceability
-
Manage communications: Choose which team members are responsible parties, pre-assign approvers and approval levels.
-
Create custom fields: Capture the right data to drive consistency. Choose between different field types, like multiple options, text, or numbers.
-
Manage anything: Build out a complete design and development package per product, product category, or even groups of products.
-
Get status updates: Get automatic updates to design data, so everyone is on the same page.
-
Build out complete design & development packages: Link with multiple modules to build out complete design and development packages per product, product category or groups of products.
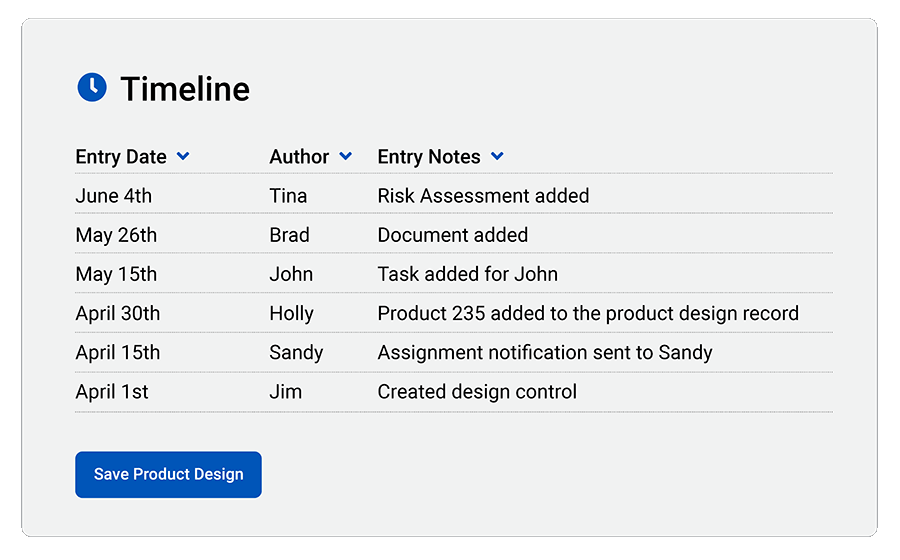
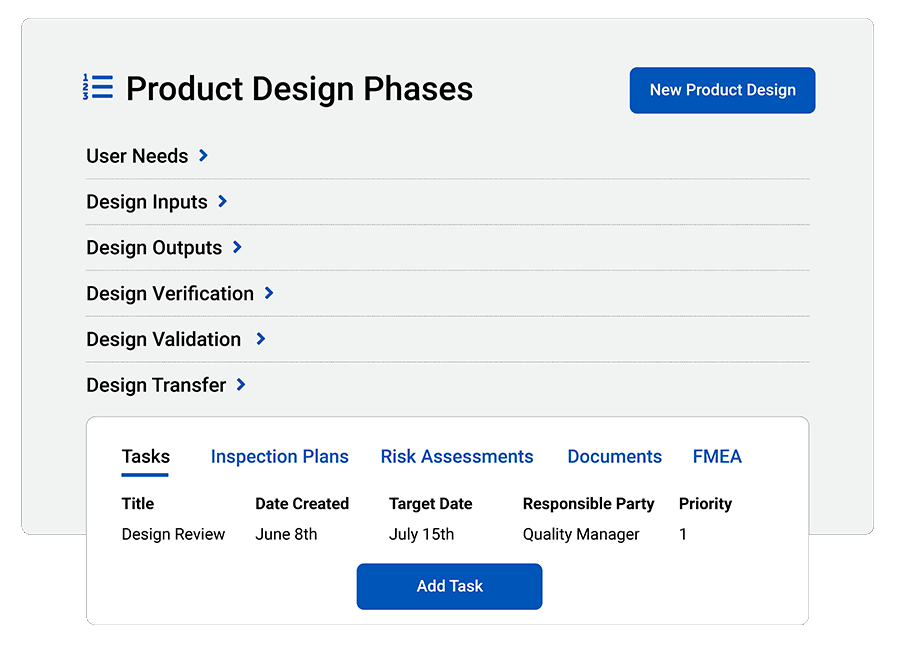
Automate compliance
-
Create Steps/Phases: Build custom steps/phases to match product design compliance with any standard.
-
FDA 21 CFR Part 820 and ISO 13485:2016 Compliance: Easily document and unify data for medical device FDA and ISO audits.
-
cGMP Compliance: Current Good Manufacturing Practices (cGMPs) require proof of proper handling for every step of medical device production. QT9 makes it easy.
-
FDA 21 CFR Part 11 Compliance: Review and electronically approve documents with FDA 21 CFR Part 11-compliant electronic signature approvals.
-
Maintain Design History File (DHF): Quickly prove that you have satisfied design controls for a review, inspection or audit.
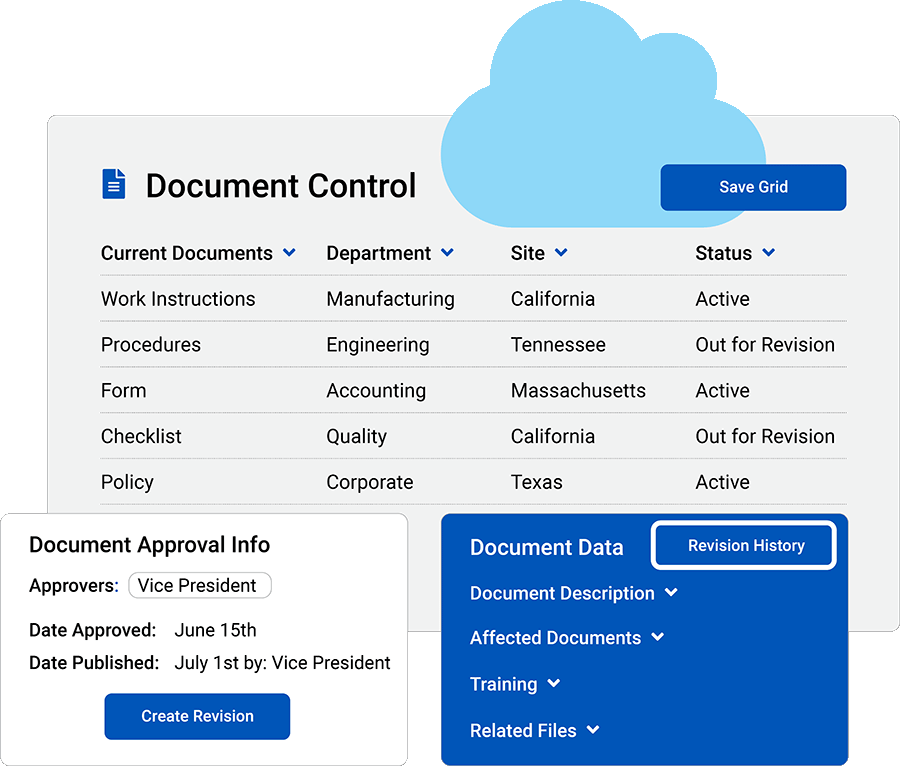
Streamline document control
-
Attach unlimited files: Access files from anywhere, and add resources so everyone is on the same page.
-
Collaborate with teams: Share read-only links that enable customers, suppliers and employees to view items securely.
-
Revision-level controls: Assign users to approve, reject or verify documents.
-
Automate training: Trigger training events by linking documents to training.
-
DHF Accessibility: QT9 makes it easy for your entire team to access Design History Files and keep them up to date.
Get an end-to-end solution for product design management
Free up time with product design controls built in to QT9 QMS.
Automate Design Controls
Templates for any design control process, including DHF and ISO 9001 requirements.
Create Custom Phases
Create custom phases of design and development.
Responsible Party
Pre-assign approvers and create responsible parties.
Ensure Compliance
Show proof of proper handling, supporting FDA Current Good Manufacturing Practices (cGMPs).
Improve Traceability
See a timeline of every action along with lot and serial traceability.
Error-Proof Production
Identify and mitigate risks associated with batch production and compliance.
Link Records
Easily link design history files to documents, audits, inspections and more.
All-in-one medical device solution
QT9 QMS makes it easy to improve your design processes and grow into fully-integrated solutions. Documentation, inspection plans and file attachments can be synced to your Bill of Materials (BOM) with QT9 ERP to create a complete eDHR or eDMR in one click.
See what quality leaders say about QT9 QMS
Get end-to-end quality management
FAQ: Design controls
Design controls are a set of quality practices and procedures that play a continual role in both premarket and postmarket medical device development.
Design controls manage the design process to assure that medical devices meet user needs, intended uses and specified requirements to improve and prevent future issues.
Design controls apply to all Class II and Class III medical devices.
Design controls apply to the following class I medical devices:
1. Devices automated with computer software
2. Tracheobronchial suction catheters
3. Surgeon's gloves
4. Protective restraints
5. Manual radionuclide applicator system
6. Radionuclide teletherapy source
Related quality management resources
Design Control Software for Medical Device Manufacturers
Try QT9 for free
Ready to simplify your quality processes? No credit card needed.