Share this
ISO 9001:2026 Transition Roadmap and QMS Guide
by Max Austin on November 06, 2025
For quality leaders, your ISO 9001:2015 Quality Management System (QMS) is the foundation for success. With the critical ISO 9001:2026 revision coming soon, having a proactive transition plan and compliance checklist is the key to maintaining compliance, reducing risk and maximizing your investment in quality.
The International Organization for Standardization (ISO) released the Draft International Standard (DIS) in September 2025. It is currently under review by member bodies, with final ISO 9001:2026 publication expected late 2026.
While the full text is still technically in development, organizations can already begin outlining their ISO 9001:2026 transition plan and preparing a readiness checklist around key areas such as quality culture, leadership engagement, sustainability and digitalization.
For quality-focused companies, this is more than a compliance update. It’s an opportunity to modernize systems and reinforce continuous improvement.
QT9 QMS offers a straightforward way to get ahead by centralizing processes, automating compliance and simplifying the transition.
This guide provides a phased ISO 9001:2026 transition plan, focusing on anticipated changes and showing how an integrated QMS like QT9 QMS gives you an essential head start toward compliance.
Contents
Understanding ISO 9001:2026 changes
A pragmatic, phased ISO 9001:2026 transition roadmap with QT9 QMS
How QT9 QMS simplifies the journey
Next steps for quality leaders
Understanding ISO 9001:2026 changes
Although the International Organization for Standardization (ISO) is still finalizing details, the working group ISO/TC 176/SC 2 has shared early direction through draft documents and committee updates. Based on these, companies can expect:
Emphasis on leadership, ethics and quality culture
Quality will move further beyond documentation. ISO 9001:2026 will likely require more visible management involvement, employee engagement and demonstration of a shared quality mindset throughout the organization.
Integration of sustainability and ESG concepts
The 2024 climate amendment to ISO 9001:2015 introduced environmental context into clause 4. The 2026 update is expected to expand on this, asking companies to evaluate how their QMS contributes to sustainability and climate impact.
Risk-based thinking and supply-chain resilience
New clauses will reinforce business continuity, supply-chain resilience and ethical practices as extensions of risk management. This is especially critical in life sciences and aerospace sectors where disruptions can affect safety and compliance.
Digital transformation
The revision acknowledges the shift toward digital QMS platforms that enable real-time data visibility, automation and analytics. Many organizations are moving toward digitizing QMS processes ahead of the revision.
QT9 Q-Cast guest and ISO expert Lorraine Caputo breaks down what's changing in ISO 9001: 2026, what's not and how you can prepare today.
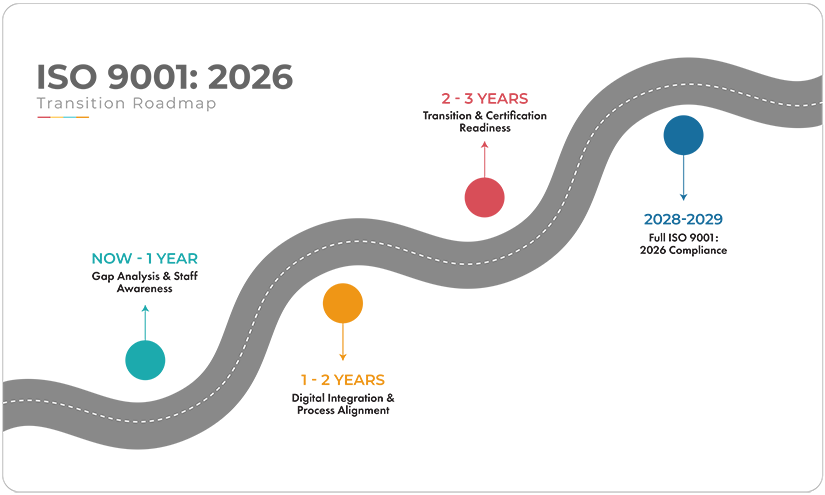
A pragmatic, phased ISO 9001:2026 transition roadmap with QT9 QMS
A structured approach to standard updates will help make the transition manageable. Here’s how organizations can use QT9 QMS to build readiness before certification bodies mandate it.
Find your baseline: Where your QMS stands today
If your company is already certified to ISO 9001:2015, this is your foundation. You already have:
-
A documented QMS with established procedures, work instructions, and records.
-
A history of internal and external audits that confirm system conformity.
-
A risk-based thinking approach embedded in core processes.
The ISO 9001:2026 revision is not a complete overhaul, it builds upon the Annex SL structure you know, focusing on modern business challenges.
Phase 1: Gap analysis and awareness (Now – 12 Months)
Begin by mapping your current system to the new standard using this ISO 9001:2026 compliance checklist.
-
Educate internal teams on expected ISO 9001:2026 focus areas: leadership accountability, sustainability and digital readiness.
-
Conduct a gap analysis using QT9’s Audit Management and Risk Management modules to assess alignment with the evolving standard.
-
Create baseline reports showing clause coverage, risk exposure and process maturity.
Early drafts and amendments confirm the key areas where the new standard will place greater emphasis. Your gap analysis should focus on these five core areas:
ISO 9001:2026 Transition checklist key areas of change
Pro Tip: QT9's configurable audit templates can be aligned to ISO 9001:2026 draft clauses, helping you start benchmarking early and track progress automatically.
Phase 2: Digital integration and process alignment (12 - 24 months)
-
Centralize documentation, training records, CAPAs and supplier data within QT9 QMS.
-
Use Document Control to automate versioning, routing and approvals, ensuring every update meets revision traceability requirements.
-
Connect objectives, risks, and supplier performance inside the platform to prepare for the new performance-based approach.
Extra Benefit: If your organization also uses QT9 ERP, integrating production and quality data creates full traceability from material sourcing to product release, an increasingly valuable proof point during ISO audits.
Phase 3: Transition and certification readiness (24 Months – 3 Years)
-
Perform internal audits based on the new structure using QT9’s built-in checklists.
-
Use Management Review dashboards to demonstrate leadership involvement through data-driven insights.
-
Assign and verify employee awareness training on new requirements through the Training Module.
-
Generate digital records and reports automatically to satisfy certification-body documentation expectations.
QT9’s automation and cloud accessibility ensure that once the new standard takes effect, updates are traceable, reports are ready, and every action is logged.
How QT9 QMS simplifies the journey
Transition Challenge |
How QT9 QMS Helps |
|
Managing manual document updates |
Automated version control and electronic approvals |
|
Maintaining leadership engagement |
Real-time dashboards and performance metrics |
|
Ensuring cross-department visibility |
Connected CAPAs, audits and risk records in one system |
|
Tracking ESG and supplier performance |
Configurable fields for sustainability and supply-chain data |
|
Demonstrating digital readiness |
Secure, cloud-based access with complete audit trails |
With QT9 QMS, organizations can move from reactive compliance to proactive improvement, aligning directly with ISO 9001’s intent to drive continual enhancement rather than checklist adherence.
Next steps for quality leaders
-
Run a readiness audit using QT9 QMS to identify process gaps and digital opportunities.
-
Map procedures against anticipated ISO 9001:2026 themes.
-
Engage leadership in regular management reviews driven by live data dashboards.
-
Digitize documentation and training to reduce future manual workload.
-
Plan a transition timeline — certification bodies typically allow a two- to three-year transition window from final publication.
Conclusion: Lead the transition
The upcoming ISO 9001 revision represents more than another compliance deadline. It’s a signal that quality management is evolving toward culture, sustainability and technology — exactly where high-performing organizations are headed.
By using QT9 QMS, your team can start preparing now with a clear ISO 9001:2026 transition plan, simplifying compliance while enhancing visibility, accountability and continuous improvement.
And if you integrate QT9 ERP, you gain an added layer of traceability across production, purchasing and quality, positioning your business ahead of competitors when ISO 9001:2026 arrives.
Ready to start your ISO 9001:2026 transition?
Schedule a QT9 QMS demo today to see how automation simplifies compliance.
FAQs: ISO 9001:2026
ISO 9001:2026 is the upcoming revision to the ISO 9001:2015 Quality Management System (QMS) standard. It updates the 2015 version to reflect modern business priorities, such as leadership engagement, quality culture, sustainability and digitalization.
The International Organization for Standardization (ISO) released the Draft International Standard (DIS) in September 2025. Following member review and final approval, the official ISO 9001:2026 publication is anticipated in late 2026.
Expected updates include:
- Greater emphasis on leadership, ethics and quality culture
- Integration of sustainability and ESG considerations
- Enhanced focus on supply-chain resilience and risk-based thinking
- Acknowledgment of digital transformation and QMS automation
Organizations typically have a 2 to 3-year transition window after the new standard is published. Certification bodies will establish specific transition timelines once ISO 9001:2026 is officially released.
QT9 QMS simplifies the transition by automating document control, risk management, training and audit management. It centralizes quality processes and provides real-time dashboards for leadership visibility, ensuring alignment with the new ISO 9001:2026 structure and requirements.
- Conduct a gap analysis against anticipated ISO 9001:2026 updates.
- Educate teams on new focus areas, such as sustainability and leadership accountability.
- Digitize documentation and processes using QT9's centralized QMS.
- Engage management in regular data-driven reviews.
- Begin integrating supply-chain and ESG data into performance metrics.
Yes. Certificates under ISO 9001:2015 will remain valid during the official transition window, expected to last two to three years after the ISO 9001:2026 final publication. Organizations should plan to transition before certification bodies stop recognizing the 2015 version.
Yes. Building on the 2024 climate amendment, ISO 9001:2026 is expected to expand sustainability expectations. It will ask organizations to consider environmental impact, stakeholder expectations and how their QMS supports climate resilience and ESG goals.
The new standard recognizes digital QMS platforms as key enablers of compliance and continuous improvement. Automated systems like QT9 QMS support real-time visibility, data analytics and traceable performance metrics, aligning directly with ISO 9001:2026’s digital focus.
Industries with complex supply chains and regulatory oversight, such as life sciences, manufacturing, aerospace, and medical devices, will see the greatest impact. These sectors benefit most from proactive digital integration and early transition planning.
Share this
- QT9 QMS (42)
- QT9 ERP (27)
- Manufacturing (15)
- QT9 MRP (14)
- Company News (12)
- Medical Devices (12)
- FDA Compliance (9)
- Pharmaceuticals (7)
- Inventory Management (6)
- Life Sciences (6)
- Document Control (5)
- QMSR (5)
- Aerospace & Defense (4)
- Analytics & Reporting (4)
- ISO 9001 (4)
- Supplier Quality Management (4)
- Bill of Materials (3)
- CAPA (3)
- FDA 21 CFR 820 (3)
- AS9100 (2)
- Accounting (2)
- Change Control (2)
- Electronic Batch Records (EBR) (2)
- ISO 13485 (2)
- Inspections (2)
- Audit Management (1)
- Calibration Management (1)
- Cannabis (1)
- Continuous Improvement (1)
- Cosmetics (1)
- Cybersecurity (1)
- DHF/DMR/DHR (1)
- Defense (1)
- Design Controls (1)
- EMS (1)
- EU Compliance (1)
- Employee Training (1)
- Food & Beverage (1)
- ISO 14001 (1)
- MoCRA (1)
- Quality Culture (1)
- Quality Events (1)
- Returns Management (1)
- Risk Management (1)
- Traceability (1)
- February 2026 (3)
- January 2026 (8)
- December 2025 (6)
- November 2025 (8)
- October 2025 (7)
- September 2025 (8)
- August 2025 (8)
- July 2025 (6)
- June 2025 (7)
- May 2025 (5)
- April 2025 (2)
- March 2025 (4)
- February 2025 (4)
- January 2025 (6)
- December 2024 (4)
- November 2024 (4)
- October 2024 (5)
- September 2024 (3)
- August 2024 (3)
- July 2024 (3)
- June 2024 (5)
- May 2024 (2)
- April 2024 (3)
- March 2024 (3)
- February 2024 (5)
- January 2024 (1)