Share this
Integrated QMS, ERP & MRP for 2026 Manufacturing

by Brant Engelhart on October 16, 2025
2026 is right around the corner. As manufacturing companies plan for the future, they face converging pressures from changing compliance standards, digital transformation and ongoing demand for operational efficiency and cost savings.
Manufacturing software integration, specifically QMS, ERP, MRP integration, has become critical for regulatory compliance, supply chain management and operational efficiency. This article explores how integrated quality management systems, enterprise resource planning and material requirements planning prepare manufacturers for 2026's compliance landscape.
Contents
The new rules of manufacturing compliance in 2026
Manufacturing supply chain resilience strategies
2026 manufacturing workforce shift: Digital talent
QMS, ERP, MRP integration drives efficiency
QT9 Software brings quality and operations together
The new rules of manufacturing compliance in 2026
The compliance landscape is evolving quickly, and manufacturers need to get ahead of these changes. In 2026, new standards and regulatory frameworks will be introduced, making compliance one of the biggest drivers of digital system upgrades.
ISO 9001 revisions
The next major update to ISO 9001 should be finalized by Fall 2026. Quality leaders anticipate changes that emphasize risk-based thinking, digital data management and sustainability requirements. Manufacturers must implement agile, cloud-based QMS tools that can track, update and report compliance seamlessly.
ESG reporting mandates
Environmental, Social and Governance (ESG) reporting is officially more than a buzzword. The EU will soon roll out new standards for its Corporate Sustainability Reporting Directive (CSRD). And Australia has begun phased implementation of the Australian Sustainability Reporting Standards (ASRS). ERP and QMS platforms that integrate ESG data and documentation will be essential for meeting these expectations.
Cybersecurity and data integrity
Increased connectivity in manufacturing has led to an increase in cybersecurity risks. New data protection mandates for manufacturing software are emerging across North America and Europe. Consolidating critical business and quality data into a single platform, rather than multiple scattered systems reduces the number of potential entry points for cyberattacks and improves visibility across the organization.
Manufacturing supply chain resilience strategies
The last few years have proven how fragile global supply chains can be. From geopolitical tensions to material shortages, manufacturers have learned that agility is necessary to keep operations running smoothly. ERP, QMS and MRP integration is an easy way to achieve supply chain agility by 2026.
Reshoring and nearshoring
North America and Europe are experiencing a wave of reshoring and nearshoring initiatives. While these efforts improve resilience, they also create complex supplier networks that require real-time coordination through ERP and MRP integration.
Geopolitical instability
Trade disputes, tariffs and regional conflicts continue to disrupt raw material sourcing. Manufacturers can no longer rely on static supplier data, they need systems that provide live updates and predictive risk management.
Demand for real-time visibility
Supply chain visibility is no longer optional. Customers expect visibility into sourcing, lead times and delivery schedules. ERP, QMS and MRP integration enables real-time dashboards that support proactive decision-making and enhance customer trust.
2026 manufacturing workforce shift: Digital talent
The manufacturing workforce has steadily undergone a vital shift, losing a significant percentage of skilled workers in the last five years. By 2026, demographic shifts, skill gaps and new employee expectations will reshape how factories operate.
Labor shortages
By 2033, 3.8 million manufacturing jobs will be needed in the United States, and 1.9 million are expected to go unfilled, according to the National Association of Manufacturers (NAM). This challenge is particularly acute in high-skill areas, such as machining and quality control. Automation and digital systems will be needed more than ever to fill the void.
Upskilling for digital systems
Current skilled-labor forecasts mean that manufacturers must invest in training and upskilling programs that prepare employees for digital-first systems. ERP, QMS and MRP platforms that are intuitive and easy to adopt will help overcome these issues.
Gen Z workforce expectations
As younger generations enter the workforce, these digital natives expect modern, user-friendly tools with real-time transparency. Manufacturers must invest in creating a work environment that appeals to the next generation, including automation and robotics. Companies still operating on clunky, outdated systems risk losing the next generation of talent.
How QMS, ERP, MRP integration drives efficiency
The greatest advantage of connecting QMS, ERP and MRP software is the ability to create one continuous thread of information across the entire organization. This level of integration reduces manual tasks, prevents inaccuracies and ensures every department operates with the same, up-to-date information.
Benefits of real-time data flow in manufacturing
When QMS, ERP and MRP software exchange information instantly, companies can respond to issues without delay. For instance, if a nonconformance is logged into the QMS, ERP software can automatically put affected purchase orders on hold or adjust production schedules. MRP integration can recalculate materials demand. This prevents costly errors from escalating downstream.
Electronic Batch Records (EBR) and Device History Records (DHR)
For industries subject to FDA, EMA or ISO regulations, maintaining accurate batch and device history records is a must. Integrated ERP and MRP systems can generate electronic batch records automatically by linking production data, materials used and operator activity. QMS software then connects these records with approved processes, ensuring complete traceability. Instead of manually reconciling paper files, auditors and regulators can view a complete, validated electronic record at any time.
Supplier and vendor management
Suppliers are the starting point of product quality. QMS software tracks supplier approvals, audits and corrective actions, while ERP software manages purchase orders, taking vendor performance metrics into account. When these systems share data, organizations gain a complete view of supplier reliability. If a supplier fails to meet requirements, ERP software can avoid that vendor for future orders.
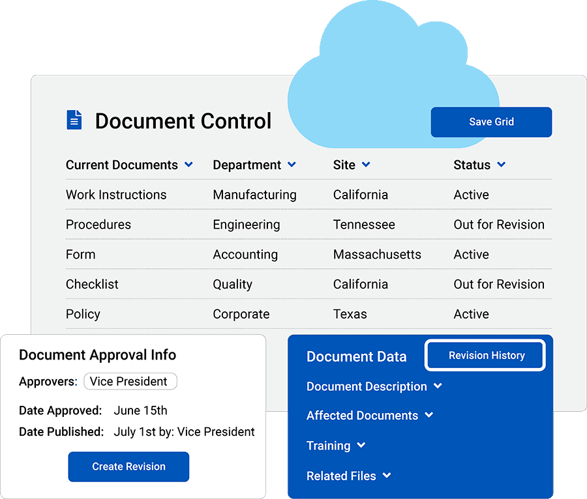
Document control and revision management
Production efficiency suffers when staff work from outdated procedures. QMS centralizes document control and revision management, ensuring employees always have access to the latest approved SOPs and work instructions. When linked to ERP and MRP, those controlled documents automatically guide purchasing and production activities. This reduces training gaps and eliminates the risk of obsolete instructions reaching the shop floor.
Customer complaints and returns
How an organization handles complaints and returns directly impacts efficiency and customer trust. Integrated QMS captures the details of a customer complaint, initiates an investigation and connects the issue to the related lot or batch in ERP/MRP. From there, nonconforming materials can be identified, production can be adjusted and suppliers can be notified if needed. The result is a faster, closed-loop process that addresses the root cause and prevents future issues.
Production and purchasing inspections
Quality control should not be left to the end of the production process. It must be built in at every step. With QMS/ERP/MRP integration, inspections performed at receiving, in-process or final stages are recorded in either the QMS or ERP/MRP and linked to ensure data is available to act upon and for compliance purposes.
If a purchased material fails inspection, ERP can automatically block its use and MRP can adjust material requirements. This proactive approach prevents wasted labor and rework while ensuring only approved materials move forward.
Reduced waste and rework
By ensuring all manufacturing inputs, such as raw materials and components, are sound, the risk of production errors diminishes significantly. Scrap and rework costs drop because nonconformances are identified early, often before they enter production. ERP and MRP keep costs and schedules aligned, while QMS ensures compliance and continuous improvement.
Faster decision-making
One of the most powerful benefits of QMS/ERP is complete visibility into all business processes as well as compliance progress. Leaders have the ability to instantly see how a supplier issue flagged in QMS affects production schedules in MRP and cost forecasts in ERP. Instead of reacting to problems after they cause delays, decision-makers can act proactively, supported by connected, accurate data.
Simplified compliance
Manufacturers in life sciences and aerospace often juggle stringent regulations with tight production demands. With integration, compliance becomes less of a burden and more of a built-in advantage. Audit trails, batch records, inspection data and supplier performance reports are all connected, making it easier to demonstrate adherence to regulatory requirements without slowing operations.
QT9 Software brings quality and operations together
QT9 Software is one of the only providers offering a seamlessly integrated QMS and ERP/MRP solution, built to work together from the start.
Because QT9 QMS and QT9 ERP are designed as complementary systems, manufacturers avoid the common pitfalls of disconnected software. Data flows automatically, compliance is simplified and efficiency gains are realized immediately.
Manufacturers in life sciences, aerospace and many other industries use QT9 to streamline processes, improve quality and reduce costs without sacrificing compliance or scalability.
FAQs: QMS ERP MRP Integration
QMS ERP MRP integration connects quality management systems, enterprise resource planning and material requirements planning into one unified platform for real-time data sharing and improved manufacturing efficiency.
Integrated systems help manufacturers meet new ISO 9001 requirements, ESG reporting mandates and cybersecurity standards while improving supply chain visibility and operational efficiency.
Integration creates automatic audit trails, connects batch records with quality data and provides real-time compliance dashboards for FDA, ISO and other regulatory requirements.
Regulated industries, such as medical devices, pharmaceuticals, nutraceuticals and aerospace, gain the most immediate benefits as they require strict compliance, full traceability and risk-based quality management. However, any manufacturer looking to improve efficiency and reduce errors can benefit from integrated quality and enterprise management systems.
With labor shortages and a growing Gen Z workforce, manufacturers need user-friendly, digital-first tools. Integrated QMS/ERP/MRP platforms automate routine tasks, reduce manual data entry and provide intuitive dashboards that empower employees to focus on value-added work rather than repetitive administration.
Related manufacturing resources

Optimize Manufacturing with In-Process Inspections

Supplier Quality 2.0: Monitor Risks & Maintain Control

QT9 ERP eBatch Records Streamline Compliance
Share this
- QT9 QMS (44)
- QT9 ERP (29)
- Manufacturing (16)
- QT9 MRP (14)
- Company News (13)
- Medical Devices (12)
- FDA Compliance (9)
- Inventory Management (7)
- Pharmaceuticals (7)
- Life Sciences (6)
- QMSR (6)
- Document Control (5)
- Aerospace & Defense (4)
- Analytics & Reporting (4)
- ISO 9001 (4)
- Supplier Quality Management (4)
- Bill of Materials (3)
- CAPA (3)
- FDA 21 CFR 820 (3)
- AS9100 (2)
- Accounting (2)
- Change Control (2)
- Electronic Batch Records (EBR) (2)
- ISO 13485 (2)
- Inspections (2)
- Audit Management (1)
- Calibration Management (1)
- Cannabis (1)
- Continuous Improvement (1)
- Cosmetics (1)
- Cybersecurity (1)
- DHF/DMR/DHR (1)
- Defense (1)
- Design Controls (1)
- EMS (1)
- EU Compliance (1)
- Employee Training (1)
- Food & Beverage (1)
- ISO 14001 (1)
- MoCRA (1)
- Quality Culture (1)
- Quality Events (1)
- Returns Management (1)
- Risk Management (1)
- Traceability (1)
- February 2026 (8)
- January 2026 (8)
- December 2025 (6)
- November 2025 (8)
- October 2025 (7)
- September 2025 (8)
- August 2025 (8)
- July 2025 (6)
- June 2025 (7)
- May 2025 (5)
- April 2025 (2)
- March 2025 (4)
- February 2025 (4)
- January 2025 (6)
- December 2024 (4)
- November 2024 (4)
- October 2024 (5)
- September 2024 (3)
- August 2024 (3)
- July 2024 (3)
- June 2024 (5)
- May 2024 (2)
- April 2024 (3)
- March 2024 (2)
- February 2024 (5)
- January 2024 (1)