ISO 13485 Compliance Made Simple
Connect and centralize your ISO 13485 compliance processes for maximum efficiency.
Automate Workflows
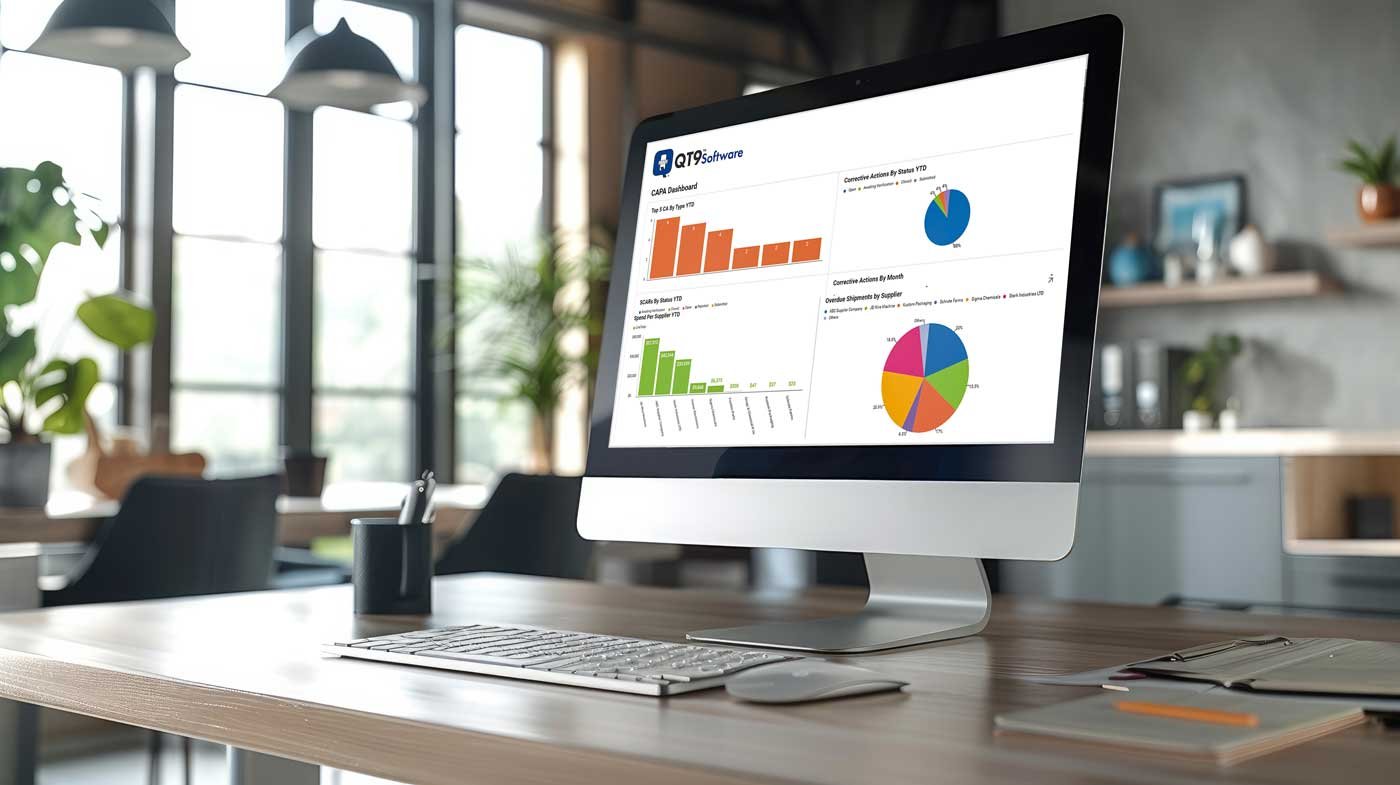
QT9 QMS and QT9 ERP automate workflows by dynamically connecting data and processes across quality and operations.
ISO 13485: 2016 Certification
QT9 helps make ISO 13485 certification and audits as easy as possible.
Connect & Collaborate Online
Engage interested parties through secure supplier and customer web portals that support collaboration and transparency.
Continuous Improvement
QT9 provides guidance and tools to maintain and continuously improve quality.
ISO 13485 compliance for the modern medical device maker
Keep track of medical device compliance items and synchronize documentation with anyone, anywhere.
Explore how ISO 13485 software can benefit your organization
Enhance operating efficiency
Simplify ISO 13485 compliance
Streamline audits and inspections
Simplify internal and external audits by automating scheduling, tracking and reporting compliance data in one central place.
Reduce waste and costs
Increase customer satisfaction

See how QT9 stacks up against the ISO 13485:2016 standard
QT9’s ISO 13485 compliance solution for the medical device industry provides a complete, centralized platform to support ISO 13485:2016 requirements.

Pre-validated QMS software
QT9 QMS is pre-validated for accelerated compliance.
-
FDA and ISO compliance out-of-the-box
-
Rapid cloud deployment for faster implementation
-
Audit-ready with timeline traceability
-
Electronic signature approvals and secure access controls
-
Continuous validation and updates
|
• ISO 13485 |
• FDA 21 CFR Part 11 |
QT9 QMS includes a complete execution of all protocols, including Installation Qualification (IQ), Operation Qualification (OQ) and Performance Qualification (PQ).
-
Eliminates costly internal QMS validation efforts that can take months of staff time or cost thousands in consultant fees.
-
Reduces downtime waiting for your QMS to be approved for use.
-
Minimizes the risk of any failed audits due to flawed validation protocols.
QT9 is available in the cloud or on-premise
Flexible deployment to match your IT strategy without compromising security or speed.
Work smarter with intelligent automation in quality and compliance
QT9 Software’s built-in automation streamlines compliance workflows, quality checks, and alerts, reducing human errors, accelerating decision-making and freeing your team to focus on higher-value work.
Speed up implementation with pre-validated software
QT9 Software offers a fully pre-validated environment, with every module and feature tested and documented for compliance. Save time, avoid costly internal validation processes and go live faster with confidence.
Ensure audit-ready traceability across all processes
Track every action, change and event in real time with QT9 Software. Timeline-based traceability supports inspections, audits and product recalls by providing complete visibility into your processes and product history.
Empower teams with secure, self-service portals
QT9 Software provides dedicated web portals for employees, suppliers and customers, enabling secure self-service access to documentation and data while streamlining collaboration across your organization.
Catch and resolve issues instantly with real-time monitoring
Monitor operations live with real-time dashboards and automated alerts using QT9 Software. Enable proactive responses to nonconformances, minimize downtime and improve compliance outcomes across your organization.
Onboard faster with an intuitive, user-friendly platform
Whether you’re a startup or an enterprise, QT9 Software’s clean, intuitive interface minimizes the learning curve, empowering users to work confidently, complete tasks accurately and stay audit-ready from day one.
FAQ: ISO 13485
ISO 13485 is the international standard outlining the quality management criteria medical device manufacturers must meet to demonstrate compliance with regulatory requirements. Developed by the International Organization for Standardization (ISO), compliance with ISO 13485 demonstrates a company’s commitment to ensuring the consistent safety, effectiveness and compliance of its medical devices.
ISO 13485:2016 is the latest iteration of the 13485 standard and is the version currently used for compliance purposes. ISO standards are reviewed every five years to ensure relevance to the current market.
ISO 13485 certification is a voluntary process that requires an organization’s quality management system to pass a third-party Medical Device Single Audit Program (MDSAP) audit. Being officially certified for ISO 13485 demonstrates to customers and regulators that your medical device products reliably meet stringent quality standards.
ISO 13485 is not mandatory in the United States; however it is mandatory in several other countries, including most of Europe, Canada, Japan and Australia. The U.S. FDA also recently published a final rule amending its medical device quality requirements to bring them more in line with ISO 13485, effective February 2026.
ISO 13485 is a quality standard specific to medical devices. It is based on the more general quality management standard of ISO 9001, however ISO 13485 is a more stringent guideline with a greater focus on patient safety. The major differences between ISO 13485 and ISO 9001 include:
- Framework for implementing risk management procedures
- Rigorous document control throughout product lifecycle
- Greater environmental safety and prevention of contamination
- Required clinical and performance evaluations
- Methods for collecting customer feedback
- Product realization has a more significant impact in 13485 and greater record requirements
ISO 13485 certification is a voluntary process that requires an organization’s quality management system to pass a third-party Medical Device Single Audit Program (MDSAP) audit. Being officially certified for ISO 13485 demonstrates to customers and regulators that your medical device products reliably meet stringent quality standards.
Explore more quality management resources

ISO 13485 Software Guidelines
FDA 21 CFR Part 820 & ISO 13485 Harmonization for Med Devices
Try QT9 for free
Ready to simplify your quality processes? No credit card needed.