Pre-Validated QMS Software by QT9 Software
QT9 QMS by QT9 Software helps teams centralize quality processes on a platform that scales with your business. Streamline audits, training and document control — implemented in under 30 days.
25+ QMS modules for complete quality management
Proven eQMS for life sciences companies
QT9 makes it easy for life sciences companies to get to market faster. Achieve traceability with a pre-validated platform.
.jpeg?width=1000&height=628&name=AdobeStock_189627906_Preview%20(1).jpeg)
Introducing The QT9 Q-Cast

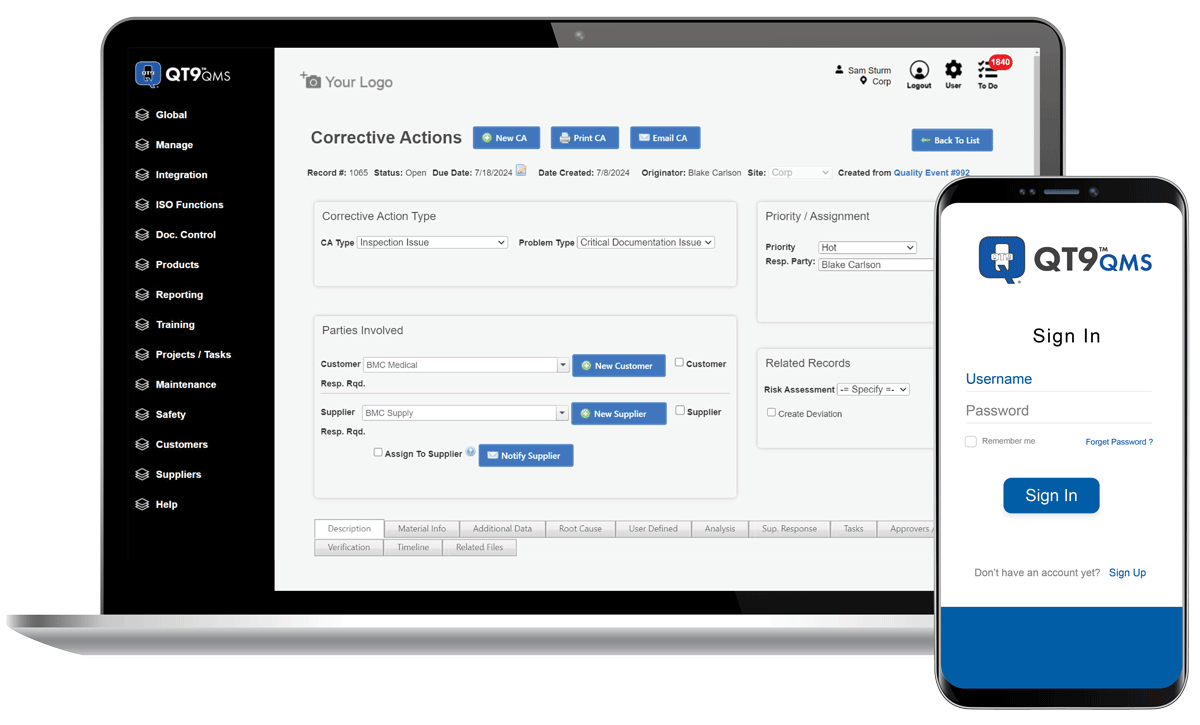
Drive quality performance across your organization
QT9 QMS enables real-time access to quality data, streamlines team communication, and automates alerts to ensure faster, smarter decisions.
Centralize quality data, information and processes in one system for easy accessibility wherever employees are located.
Invest in continuous improvement by providing a scalable, fully-automated quality system that boosts accuracy and efficiency.
Accelerate productivity and get products to market faster by streamlining quality and compliance. Dynamically populated data and automated workflows help keep projects on track.
Get the tools you need to stay agile and more easily respond to changing regulatory and market demands. Let QT9 handle the busy work, while your team focuses on innovation.

An award-winning platform. Loved by customers.
QT9 QMS is a leader in Quality Management Software on G2 — the largest software marketplace.
82%+ of compliance leaders cite third-party risks as material issues — making integrated compliance tools not just useful, but essential.
Get QT9 QMS in the cloud or on premise
Flexible deployment to match your IT strategy without compromising security or speed.
QT9 QMS next-generation automation
Get benefits beyond everyday automations with embedded workflows that enable you to work smarter and faster. Eliminate time-consuming manual quality checks and gain real-time insights to reduce human errors and speed decision-making.
QT9 QMS is pre-validated
QT9 QMS provides a pre-validated environment, enabling faster implementation and return on investment. Every module and global feature goes through a step-by-step validation. What's more, you don't have to invest in it yourself.
Provide accurate audit trails for inspections
QT9 QMS provides timeline traceability that gives transparency into every action. This makes it easier to identify defects or trace defective batches for targeted recalls.
Employee, customer and supplier portals
QT9 QMS customer, supplier and employee web portals ensure secure access to vital documents, improving communication and collaboration.
Faster issue detection and resolution
QT9 QMS enables you to identify quality issues as they occur, so you can take immediate corrective actions to minimize impact. This prevents defects, reduces downtime and minimizes compliance risks.
Reduce the learning curve
From startups to enterprises, get up to speed with a platform that’s quick to learn and easy to love. QT9 QMS helps teams minimize mistakes in data entry and workflows, ensuring accurate documentation, seamless audits and compliance with industry standards.
Pre-Validated QMS for ISO, FDA, EU MDR & AS9100
Simplified international medical device quality compliance.

EU MDR Compliance, Simplified

Built-in electronic documentation compliance with full traceability

End-to-end U.S. pharmaceutical compliance

Simplified compliance for medical devices in the U.S.

A one-stop solution for meeting ISO 9001 requirements

A streamlined environmental management system

Streamline and standardize testing lab compliance.

Accurate and efficient aerospace and defense compliance

Centralize and simplify automotive IATF 16949 compliance

Meet food safety regulations with accuracy and efficiency

Food safety compliance throughout the supply chain

QMS features that give your team a competitive edge
Expand QT9 QMS with built-in modules designed to fit your unique workflows.
Centralize Documents
Access documents in one place for better document management.
Streamline Audits
Track and manage scheduling details and the results of your audits.
Monitor Deviations
Easily track your temporary deviations with email alerts and to-do lists.
Minimize Risk
Manage risk analysis by assigning risk score, tasks, priorities, approvals, etc.
Modernize Inspections
Capture inspection details online and link related documents for reference.
Track Engineering Changes
Control ECR/ECN by assigning tasks, email alerts and electronic approvals.
Train Employees
Manage training online for employees and job roles with personalized tests.

Pre-validated QMS software
QT9 QMS is pre-validated for accelerated compliance.
-
FDA and ISO compliance out-of-the-box
-
Rapid cloud deployment for faster implementation
-
Audit-ready with timeline traceability
-
Electronic signature approvals and secure access controls
-
Continuous validation and updates
|
• ISO 13585 |
• FDA 21 CFR Part 11 |
QT9 QMS includes a complete execution of all protocols, including Installation Qualification (IQ), Operation Qualification (OQ) and Performance Qualification (PQ).
-
Eliminates costly internal QMS validation efforts that can take months of staff time or cost thousands in consultant fees.
-
Reduces downtime waiting for your QMS to be approved for use.
-
Minimizes the risk of any failed audits due to flawed validation protocols.
Why Teams Choose QT9 QMS Software
No hidden fees. No user limits. No compromises. See how QT9 QMS by QT9 Software compares to traditional quality management software.
21 CFR Part 11 Compliant
Built-in electronic signature and audit trail compliance for FDA-regulated industries.
QT9 University
Online, on-demand training resources and community forum for continuous learning.
Ready to see the QT9 difference?
Join 1,100+ companies worldwide who trust QT9 QMS software for their quality management.
Celebrated by Experts. Loved by Customers.
QT9 QMS is a leader in Quality Management Software on Capterra, Software Advice and GetApp.

See what quality leaders say about QT9 QMS
Quality management resources

Integrated QMS, ERP & MRP for 2026 Manufacturing

QT9 Earns G2 and Capterra Fall 2025 Awards

Pharma Data Integrity Updates: EU GMP Chapter 4
Try QT9 for free
Ready to simplify your quality processes? No credit card needed.
Schedule Demo
See a personalized demo of QT9 QMS with our sales team.
Start a Trial
Explore how easy QT9 QMS is able to connect operations.
Get a Quote
See how much QT9’s all-in-one platform costs.
Call QT9
Available M-F 8 AM to 5 PM CT. Speak to a QT9 expert.